Презентация на тему: Periodic table of elements

Some elements of the periodic table

Co

history of discovery 1735 Swedish mineralogist Georg Brandt

physical properties hard metal exists in two versions melting point of 1494 ° C ferromagnetic material

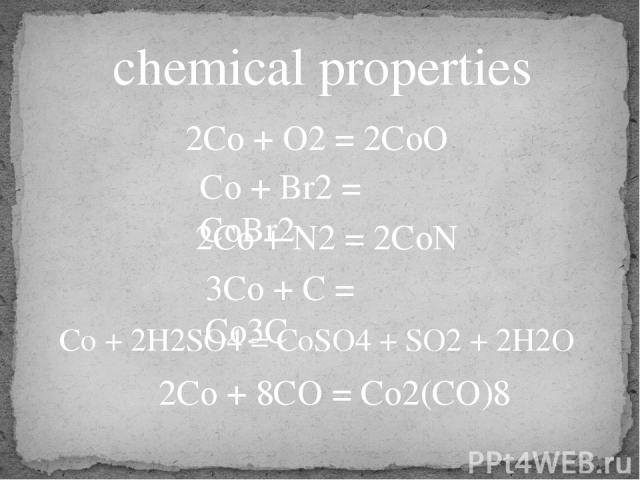
chemical properties 2Co + O2 = 2CoO Co + Br2 = CoBr2 2Co + N2 = 2CoN 3Co + C = Co3C Co + 2H2SO4 = CoSO4 + SO2 + 2H2O 2Co + 8CO = Co2(CO)8

biological function Vital for the body trace element. It is a part of vitamin B12 (cobalamin). It is involved in blood formation, function of the nervous system and liver, enzymatic reactions. The human need for cobalt 0,007-0,015 mg daily. In the absence of cobalt akobaltoz develops.

Ni

history of discovery 1751 swedish mineralogist Cronstedt

physical properties silver-white metal does not tarnish in air Has a face-centered cubic lattice In its pure form is very plastic and easy to work pressure. Density (at n. Y.) = 8.902 g / cm ³ Melting point = 1726 K

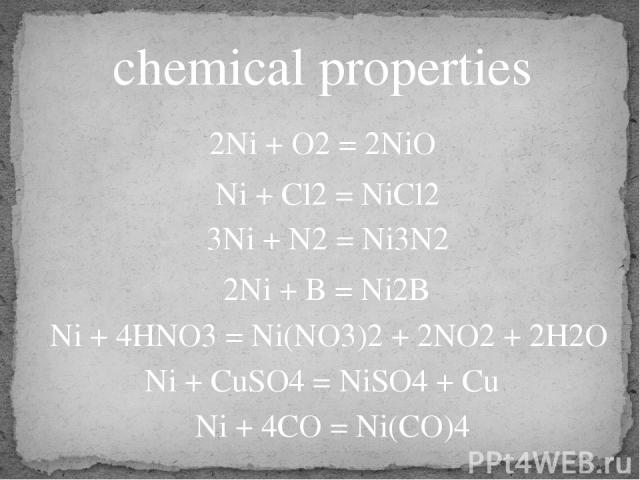
chemical properties 2Ni + O2 = 2NiO 3Ni + N2 = Ni3N2 2Ni + B = Ni2B Ni + 4HNO3 = Ni(NO3)2 + 2NO2 + 2H2O Ni + CuSO4 = NiSO4 + Cu Ni + 4CO = Ni(CO)4 Ni + Cl2 = NiCl2

biological function It is one of trace elements necessary for the normal development of living organisms. It takes part in enzymatic reactions in animals and plants. In the body, it accumulates in animal dead skin tissues, especially in the feathers.

Hf

history of discovery 1923 French chemist Jean Urbain Danish chemist: Dirk Coster and Georg de Hevesy

physical properties silvery-white, having a surface with a bright luster is not fading At ordinary temperatures it has a hexagonal lattice Density = 13.09 g/cm3 (20 ° C) refractory metal, its melting temperature = 2222 ° C One of the rare natural isotopes of hafnium, 174Hf, exhibits a weak alpha activity (half-life of 2.1015 years)

chemical properties Hf + 2F2 = HfF4 Other reactions that occur under the influence of very high temperatures and in practice. It also remains unclear their mechanism.

biological function not installed

He

history of discovery August 18, 1868 French scientist Pierre Janssen October 20, 1868 English astronomer Norman Lockyer

physical properties practically inert chemical element. nontoxic is colorless, odorless and tasteless Under normal conditions - monatomic gas Its boiling point (T = 4,215 K for 4He) the lowest of all the simple substances

chemical properties inert gas

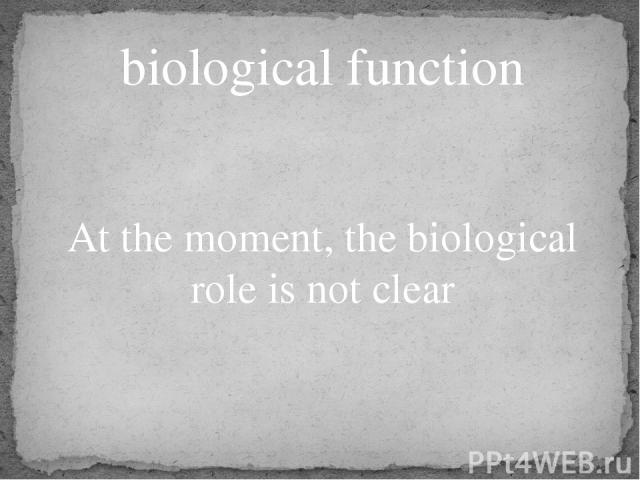
biological function At the moment, the biological role is not clear

you have viewed the presentation of some chemical elements. Slides prepared for you Kobets TimoPhEy, Department of Chemistry, the group “MXO-12"