Презентация на тему: Gene Expression Systems in Prokaryotes and Eukaryotes

Gene Expression Systems in Prokaryotes and Eukaryotes Expression studies Expression in Prokaryotes (Bacteria) Expression in Eukaryotes
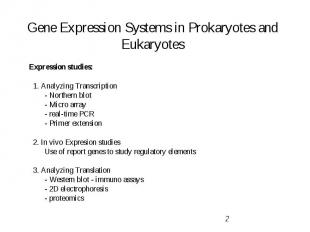
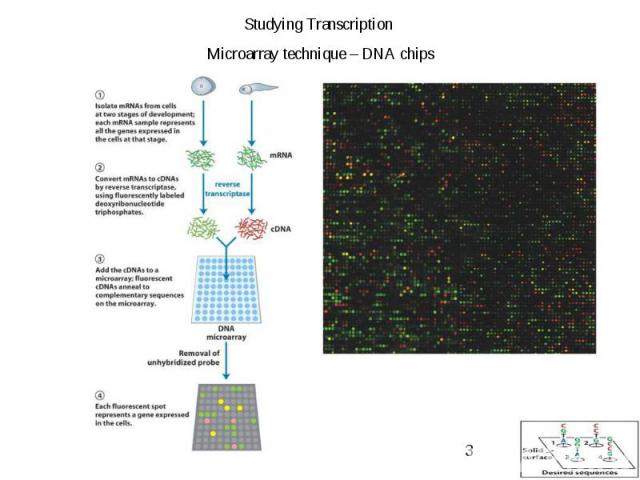
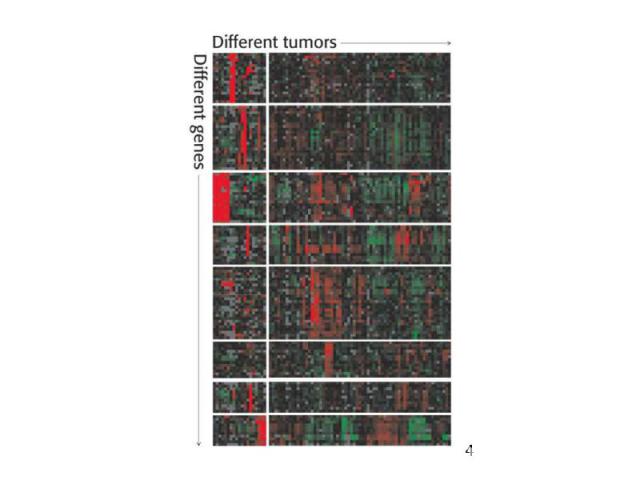
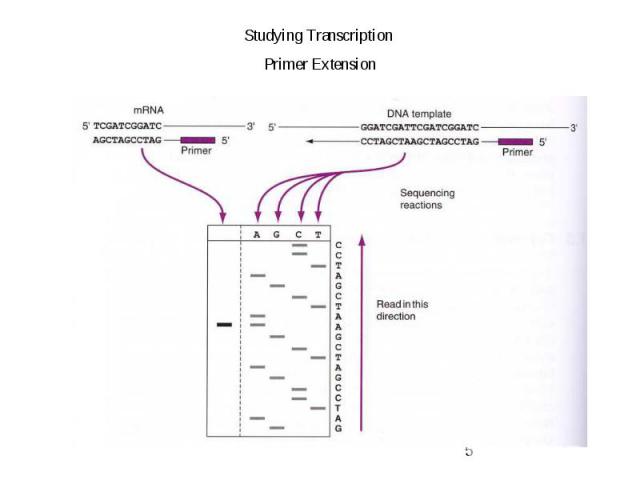
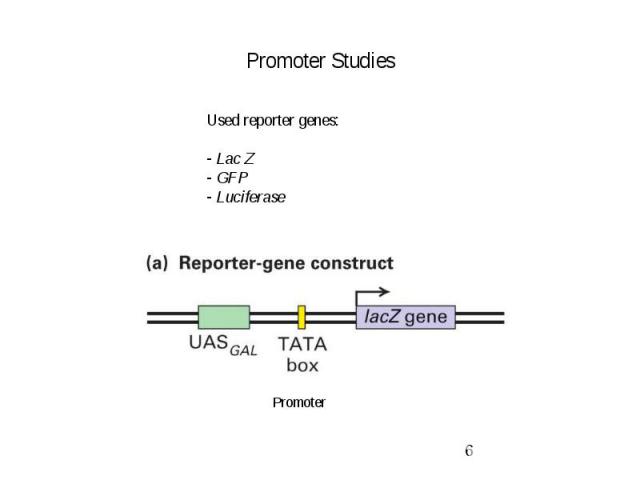
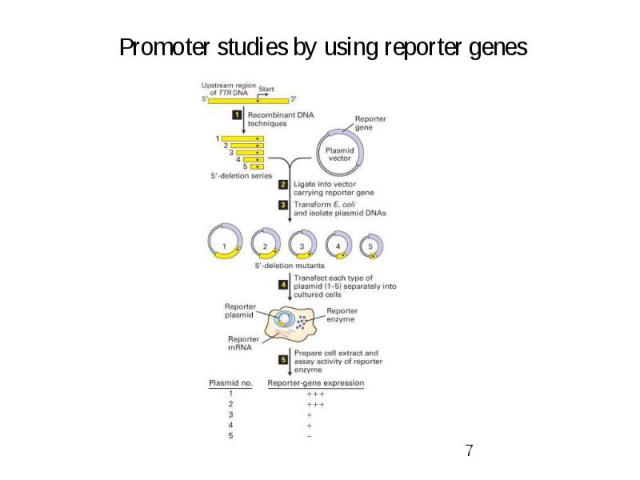
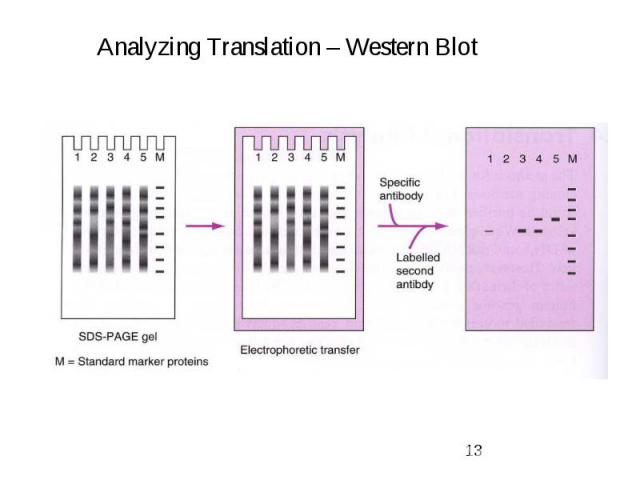
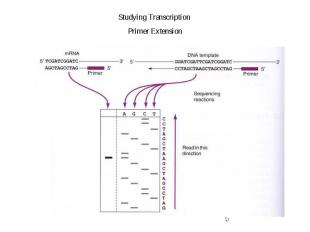
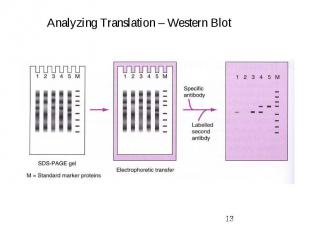
Gene Expression Systems in Prokaryotes and Eukaryotes Expression studies: 1. Analyzing Transcription - Northern blot - Micro array - real-time PCR - Primer extension 2. In vivo Expresion studies Use of report genes to study regulatory elements 3. Analyzing Translation - Western blot - immuno assays - 2D electrophoresis - proteomics

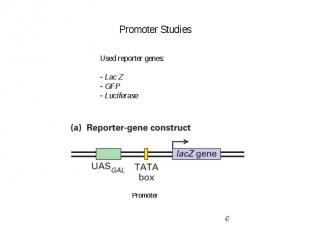
Promoter Studies

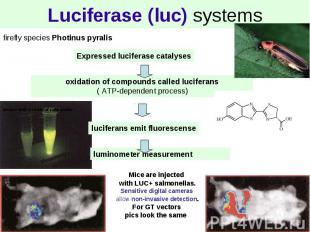
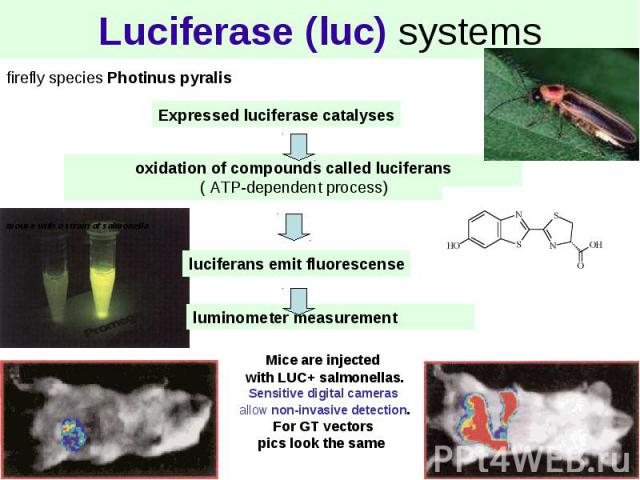
Luciferase (luc) systems

Green fluorescent protein (GFP)
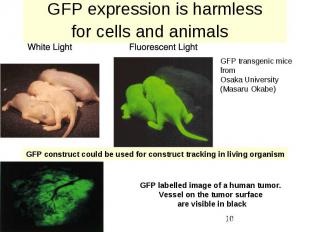
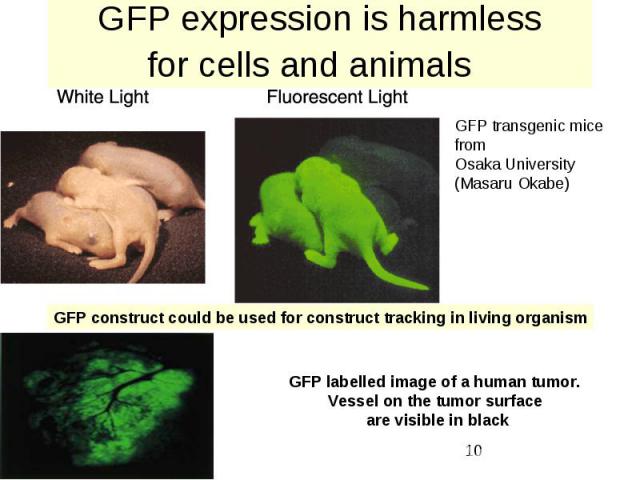
GFP expression is harmless for cells and animals
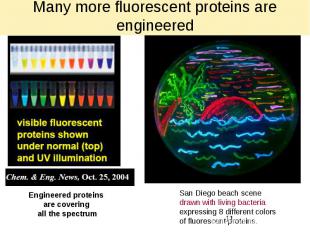
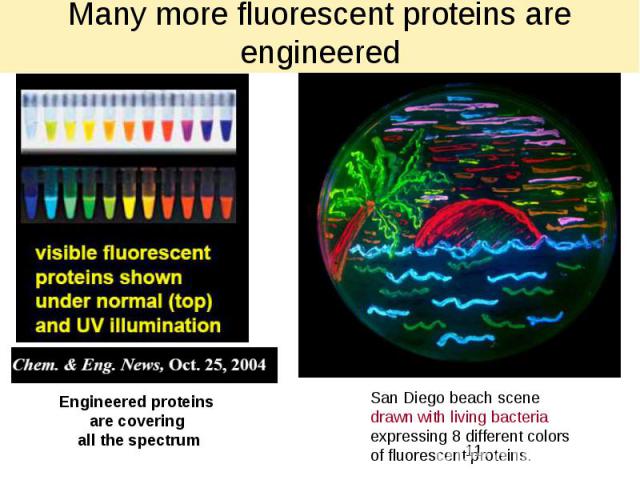
Many more fluorescent proteins are engineered
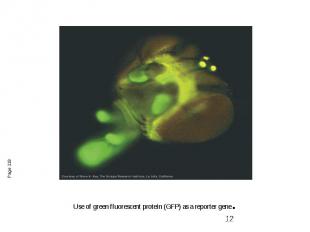
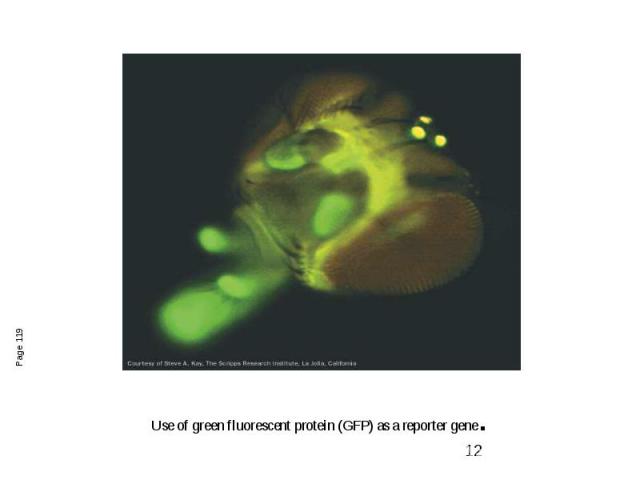
Use of green fluorescent protein (GFP) as a reporter gene.
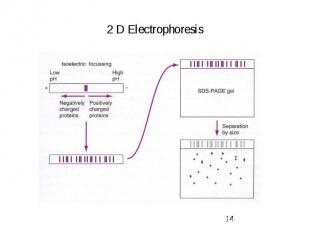
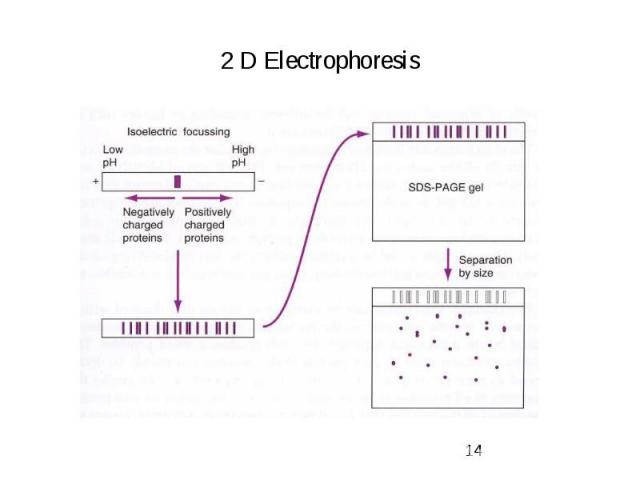
2 D Electrophoresis
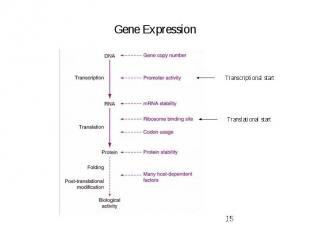
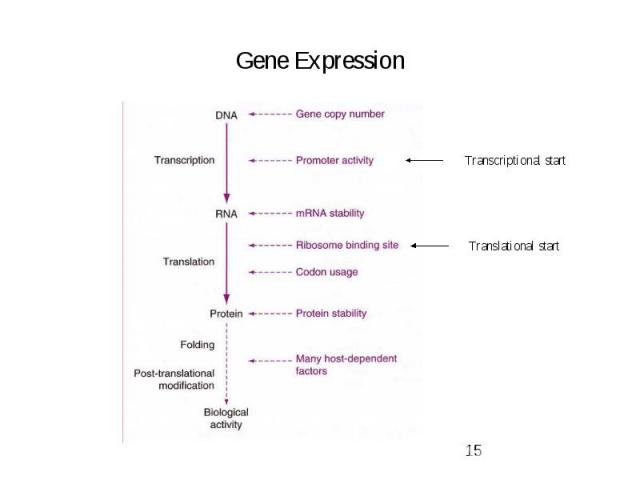
Gene Expression
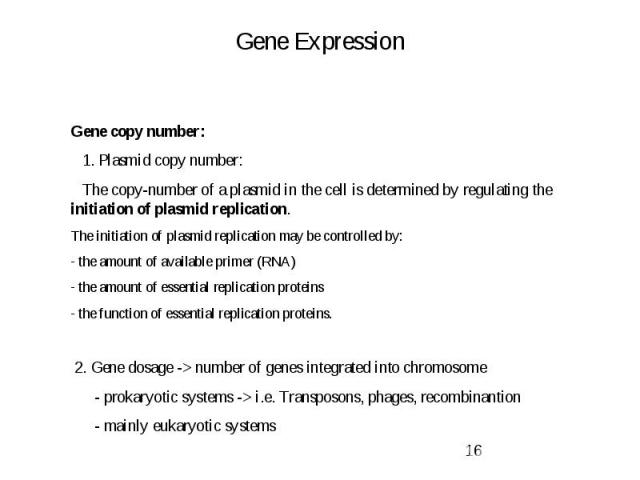
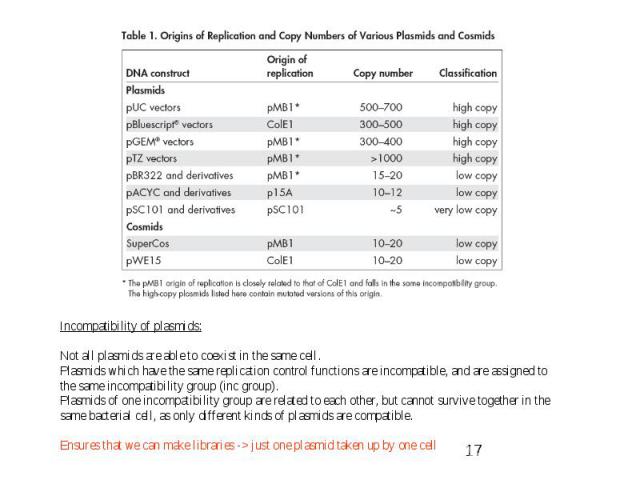
Gene Expression
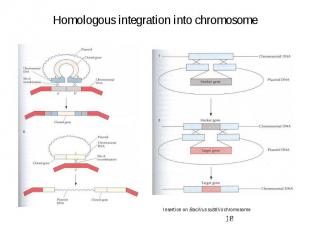
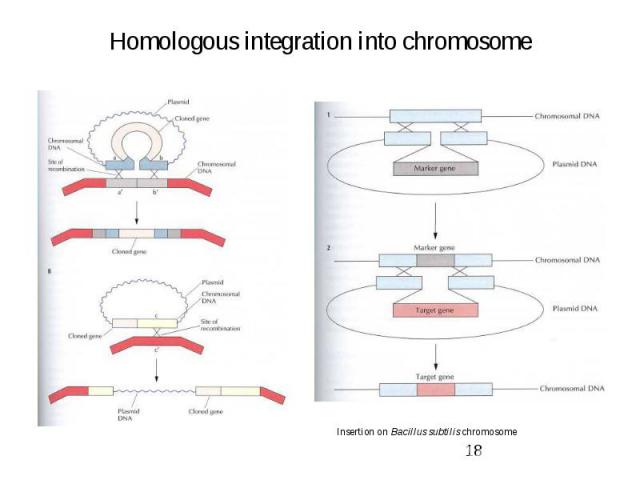
Homologous integration into chromosome
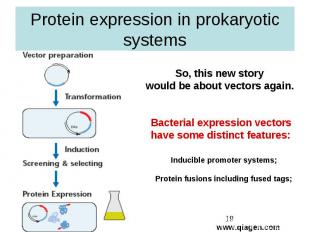
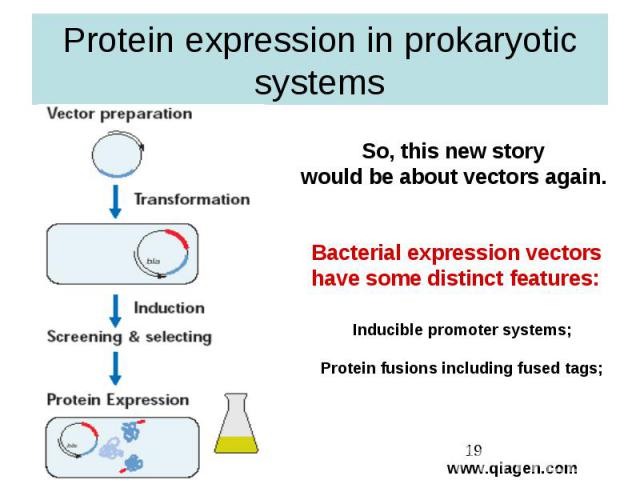
Protein expression in prokaryotic systems

General advices for one who wants to produce gene expression in prokaryotes
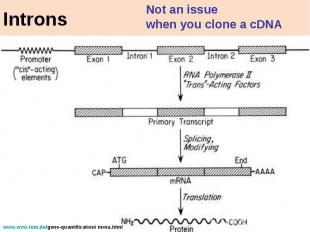
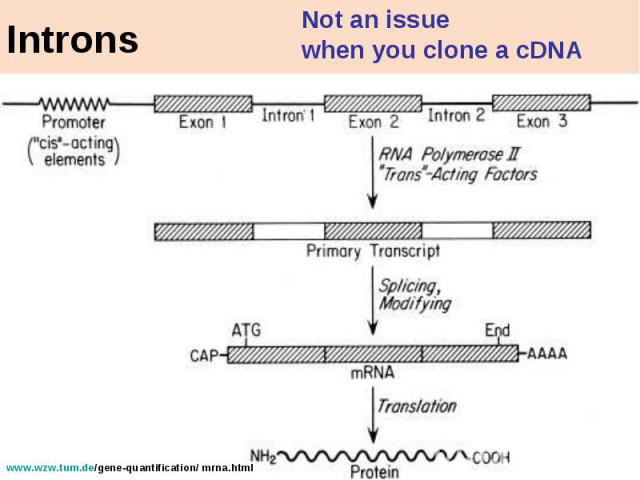
Introns
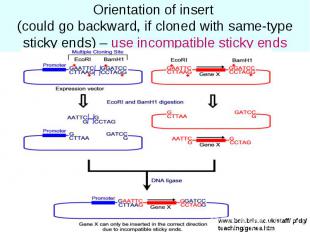
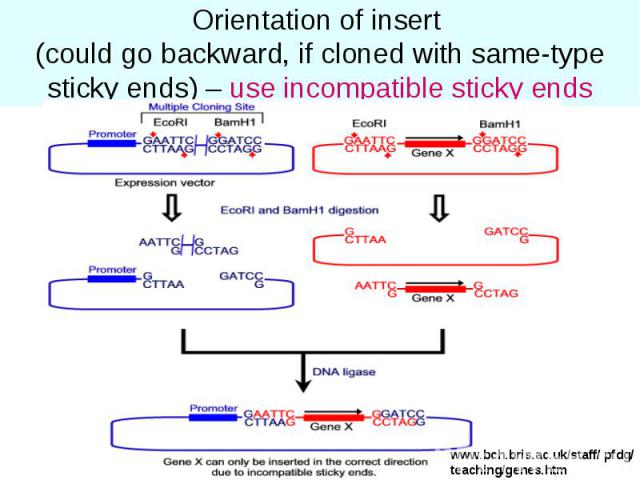
Orientation of insert (could go backward, if cloned with same-type sticky ends) – use incompatible sticky ends
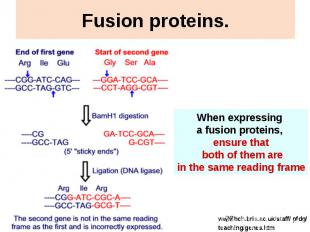
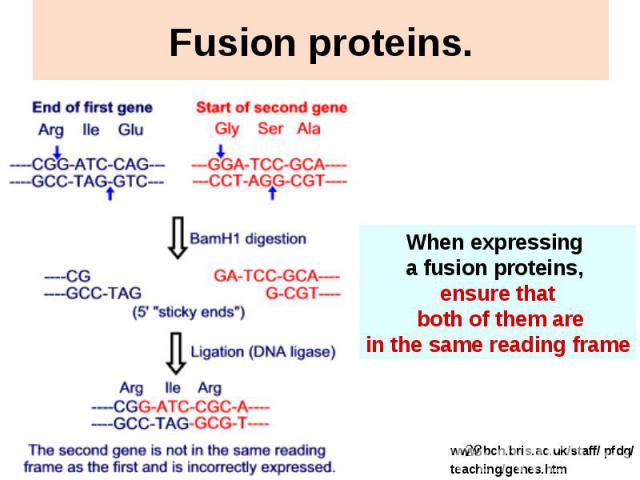
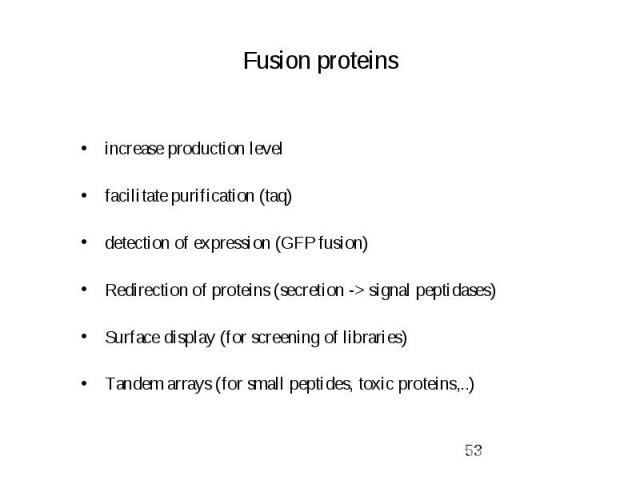
Fusion proteins.

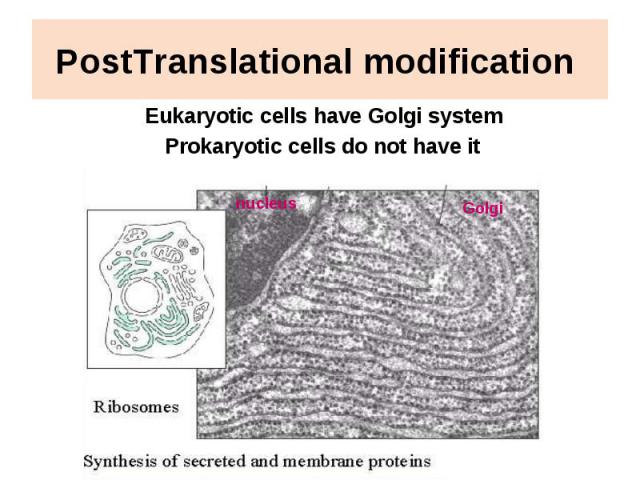
PostTranslational modification
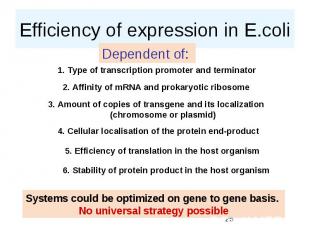
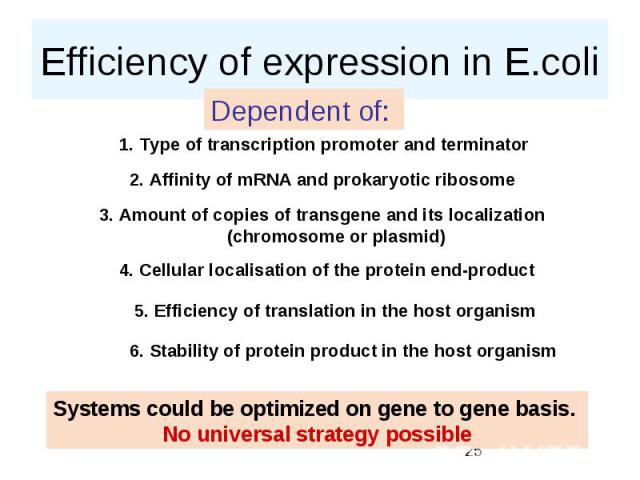
Efficiency of expression in E.coli
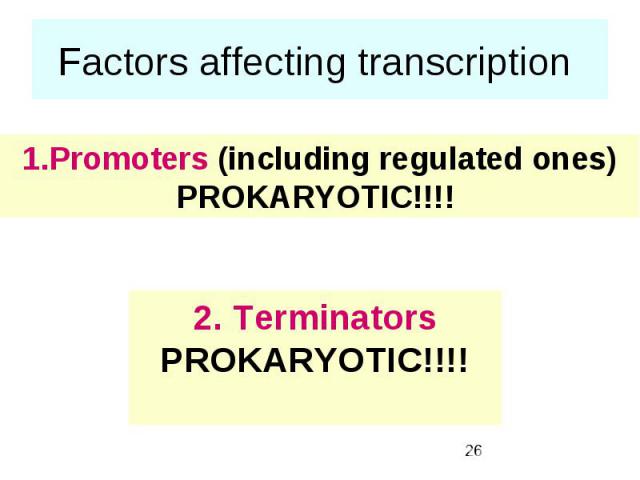
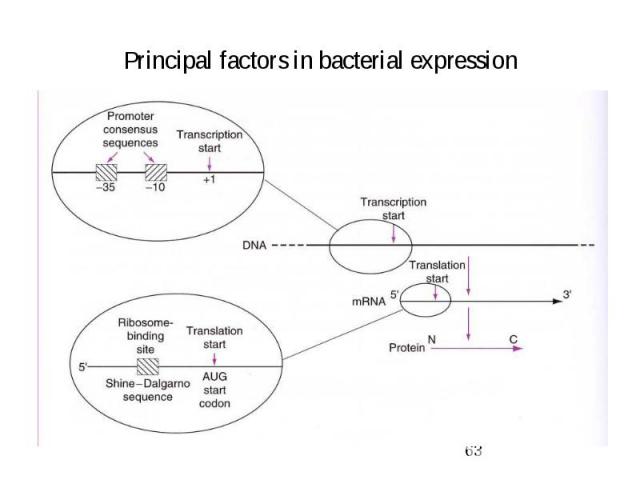
Factors affecting transcription
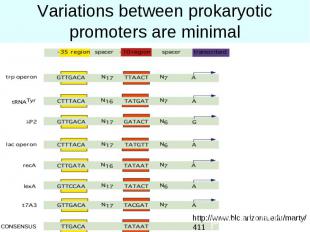
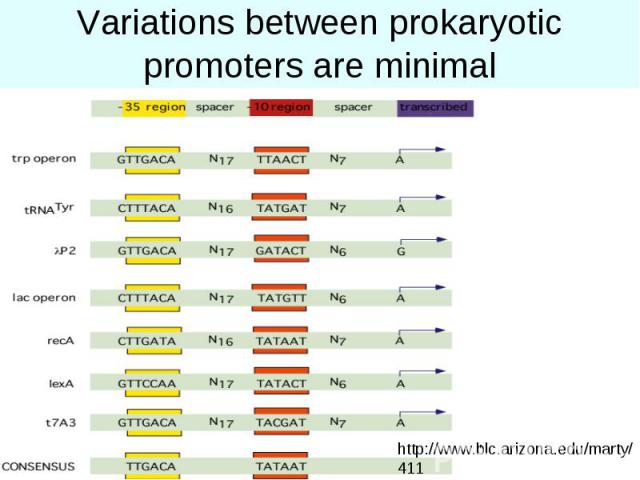
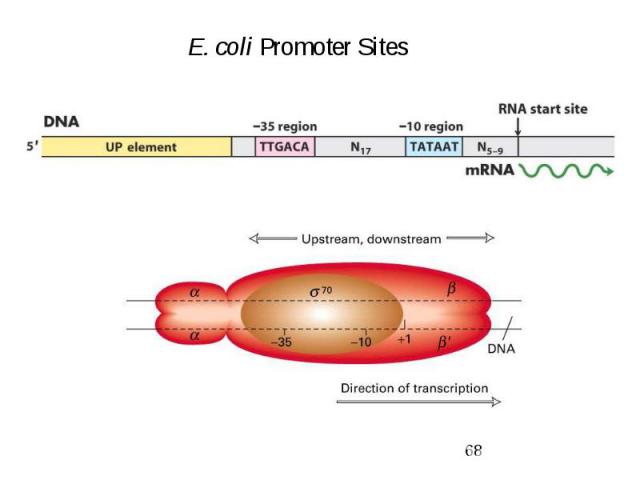
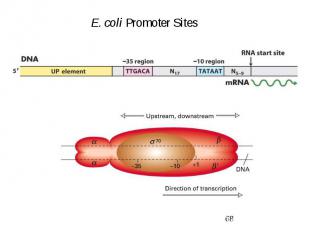
Variations between prokaryotic promoters are minimal
Factors affecting translation

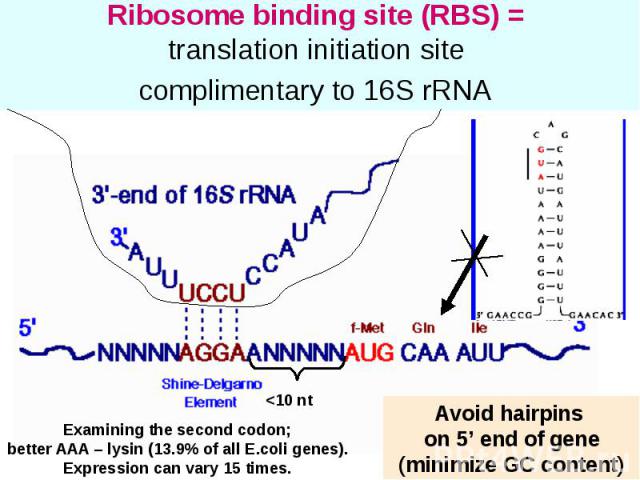
Ribosome binding site (RBS) = translation initiation site complimentary to 16S rRNA
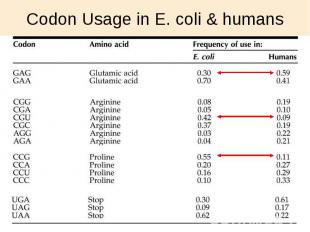
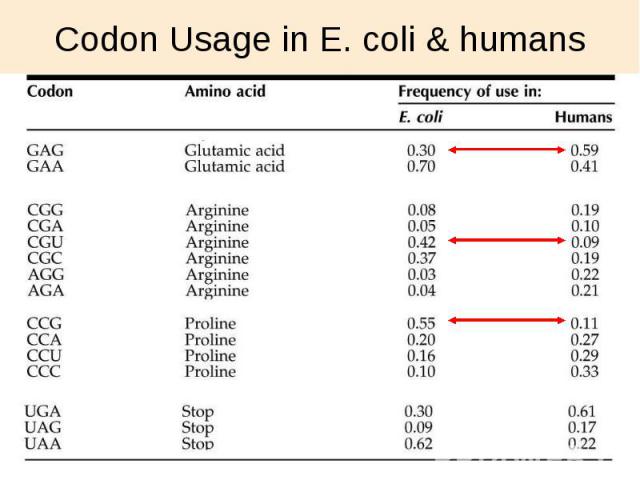
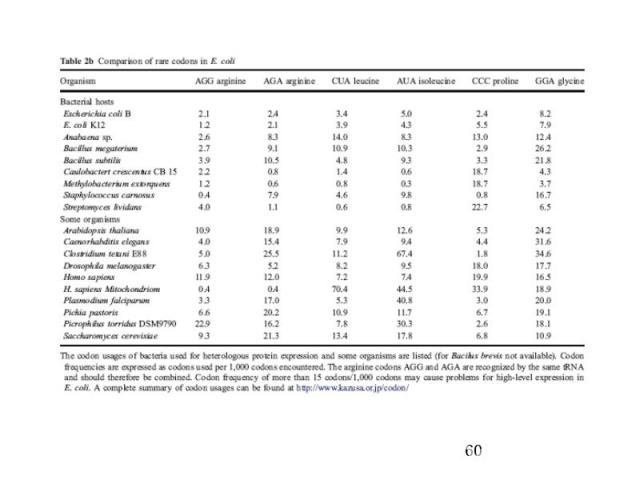
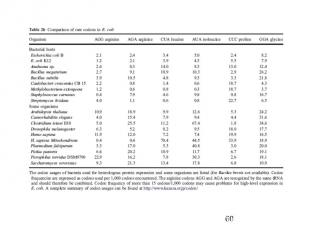
Codon Usage in E. coli & humans
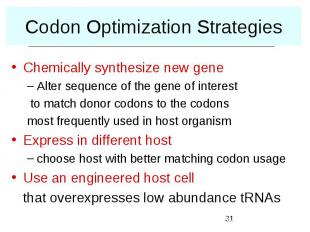
Codon Optimization Strategies Chemically synthesize new gene Alter sequence of the gene of interest to match donor codons to the codons most frequently used in host organism Express in different host choose host with better matching codon usage Use an engineered host cell that overexpresses low abundance tRNAs
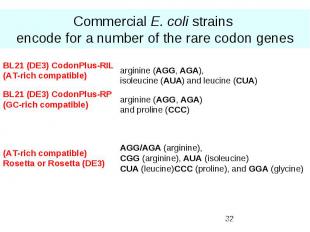
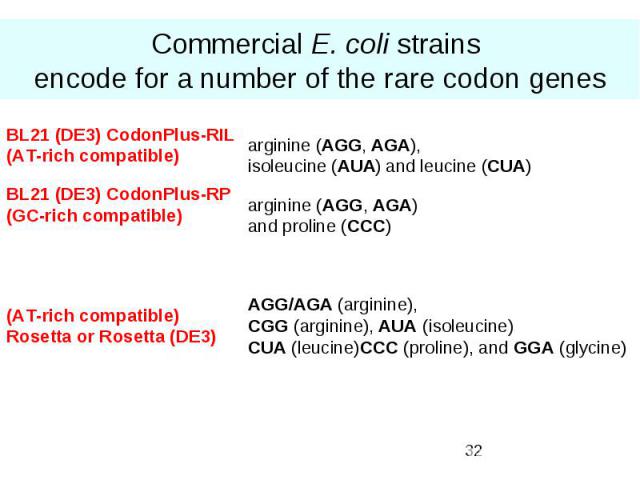
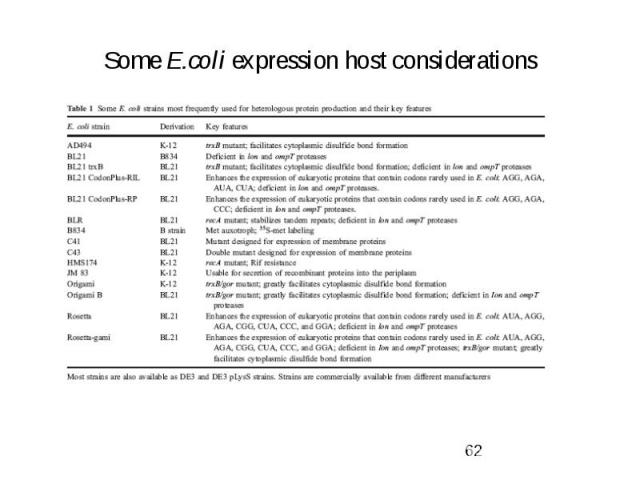
Commercial E. coli strains encode for a number of the rare codon genes
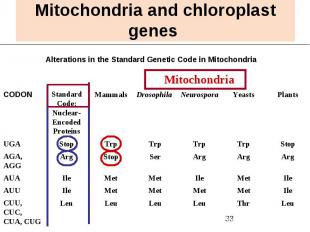
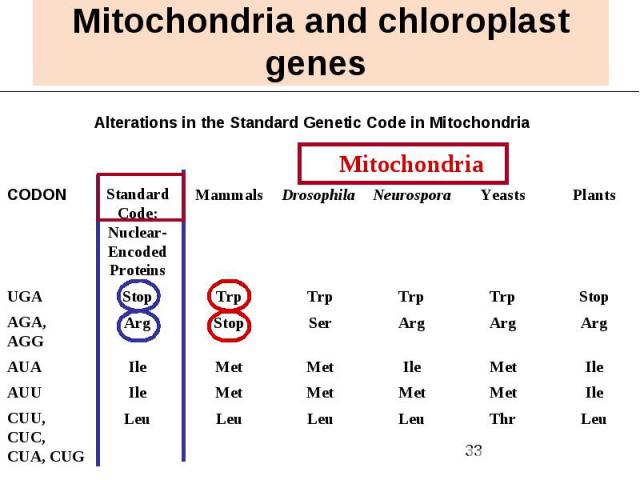
Mitochondria and chloroplast genes
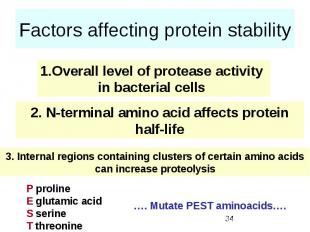
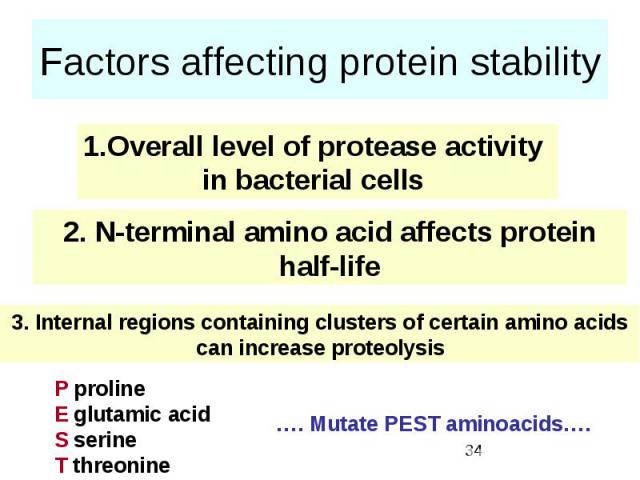
Factors affecting protein stability
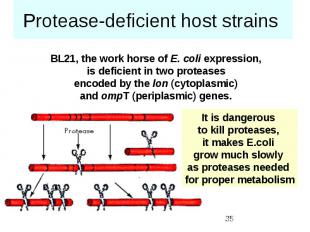
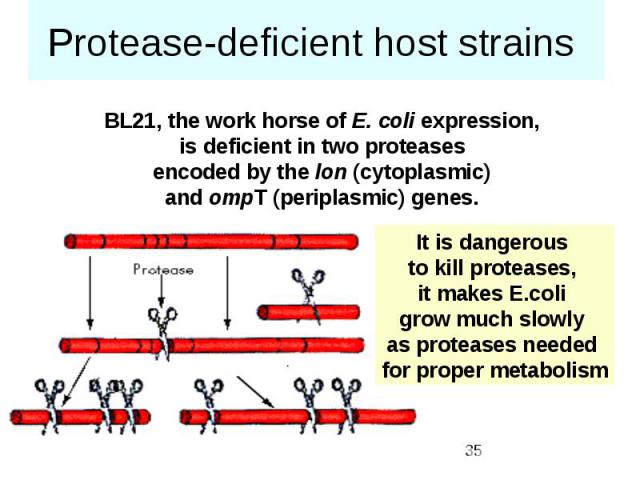
Protease-deficient host strains
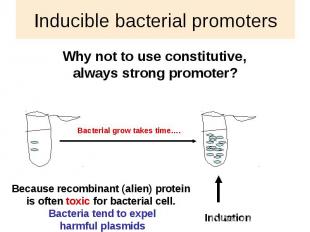
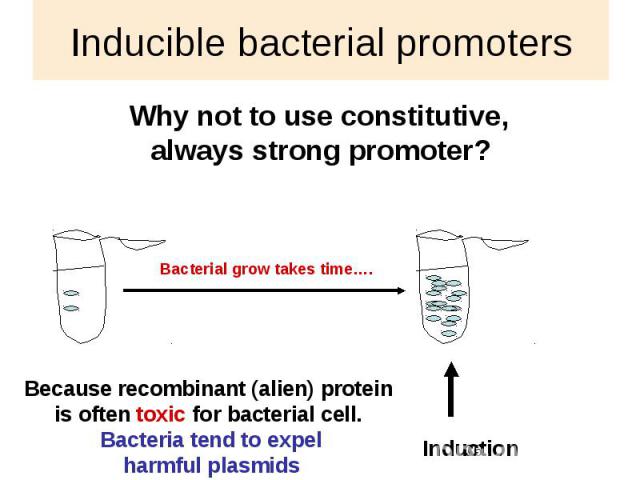
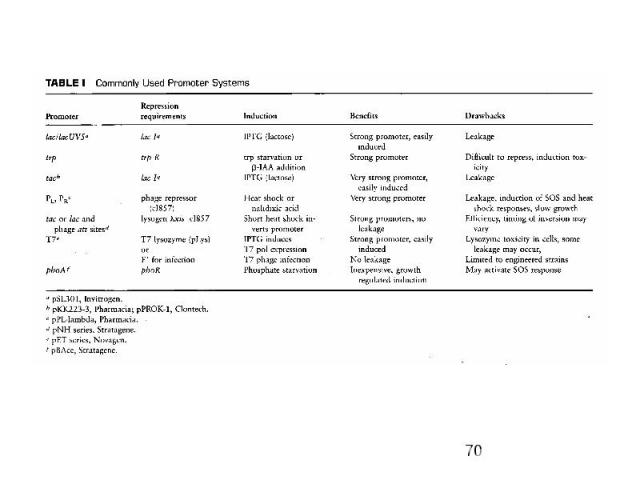
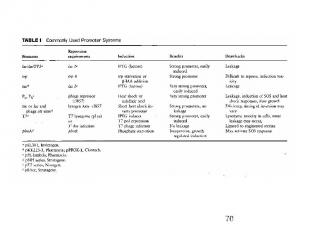
Inducible bacterial promoters
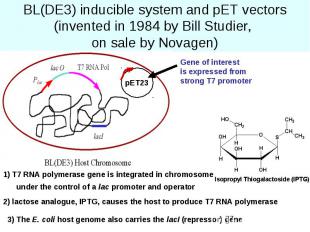
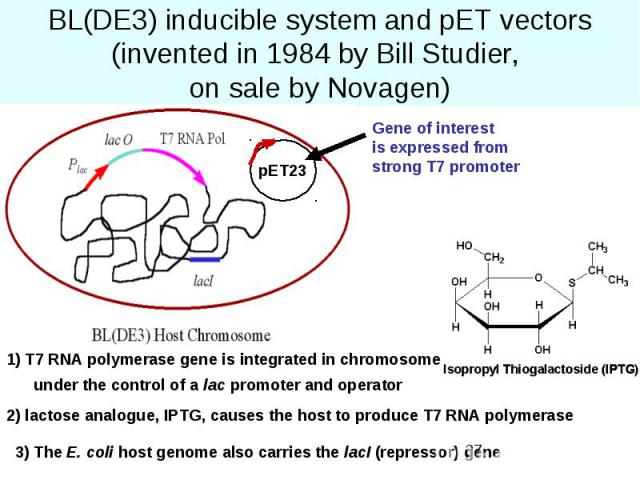
BL(DE3) inducible system and pET vectors (invented in 1984 by Bill Studier, on sale by Novagen)
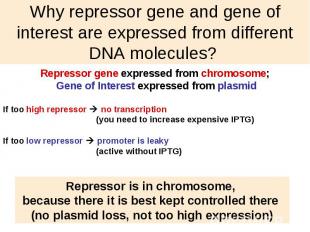
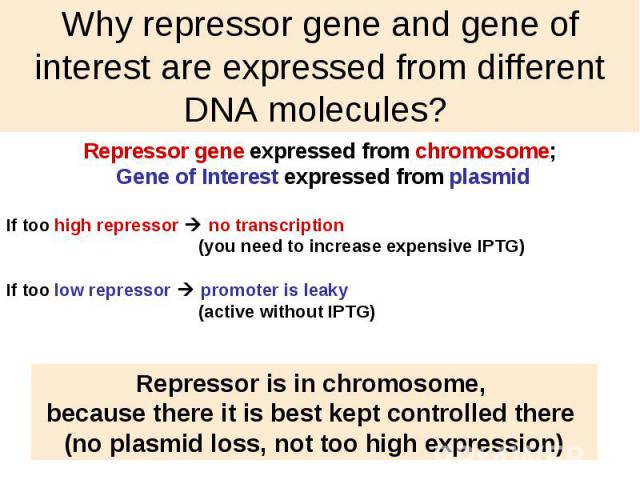
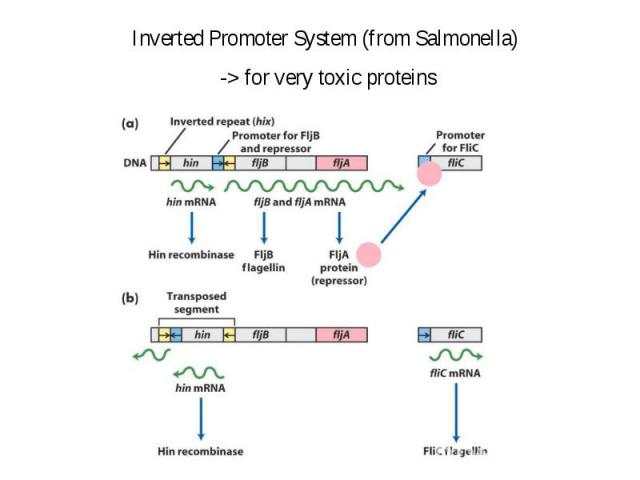
Why repressor gene and gene of interest are expressed from different DNA molecules?
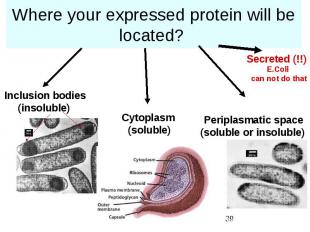
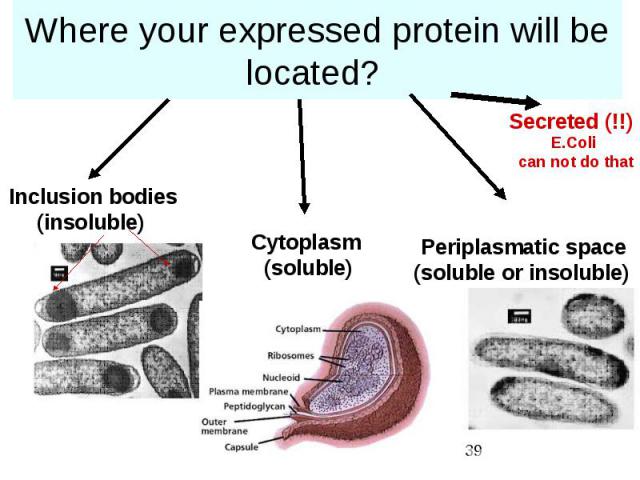
Where your expressed protein will be located?
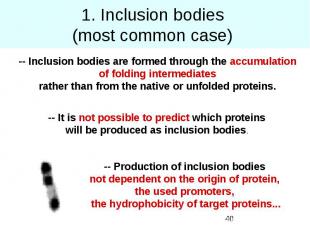
1. Inclusion bodies (most common case)
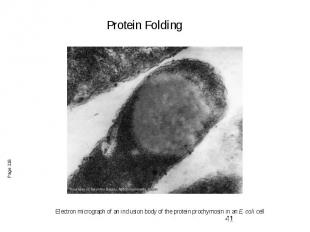
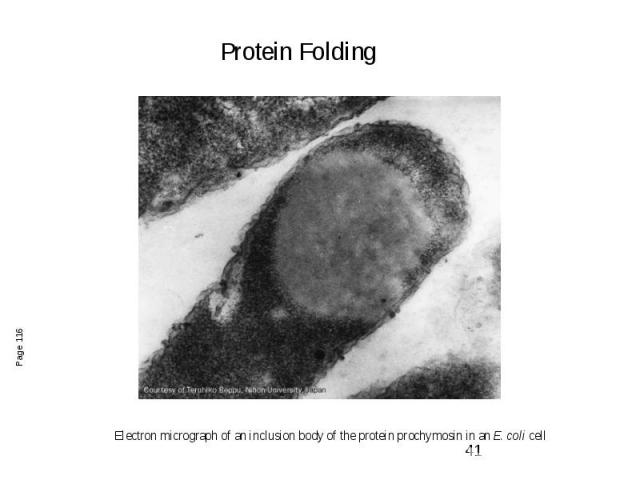
Electron micrograph of an inclusion body of the protein prochymosin in an E. coli cell

Good side of inclusion bodies
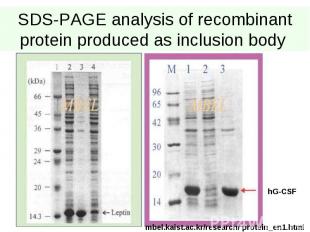
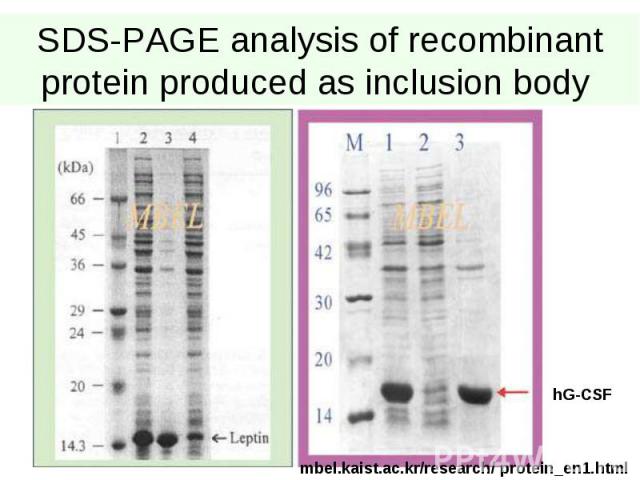
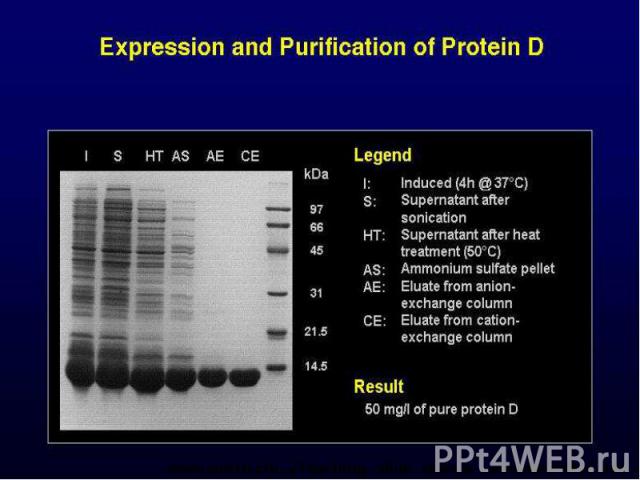
SDS-PAGE analysis of recombinant protein produced as inclusion body
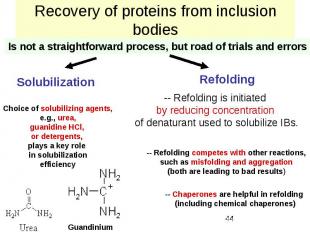
Recovery of proteins from inclusion bodies
Question of questions – how to purify your protein?
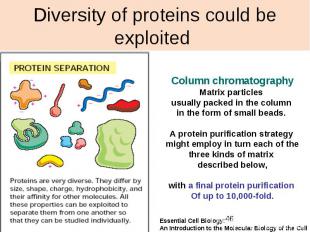
Diversity of proteins could be exploited
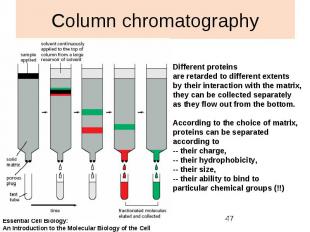
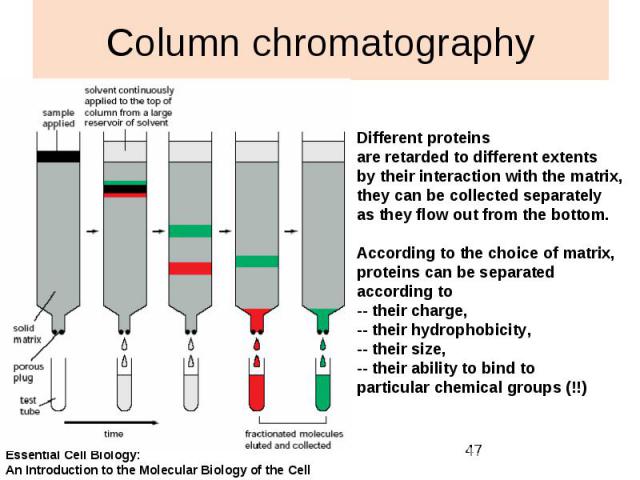
Column chromatography
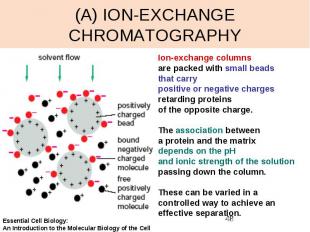
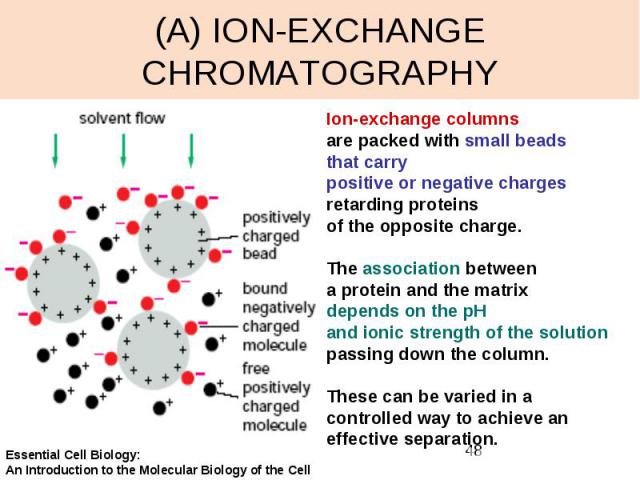
(A) ION-EXCHANGE CHROMATOGRAPHY
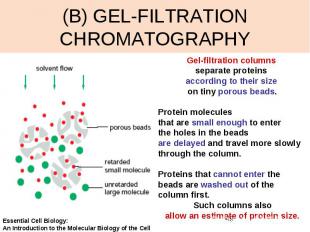
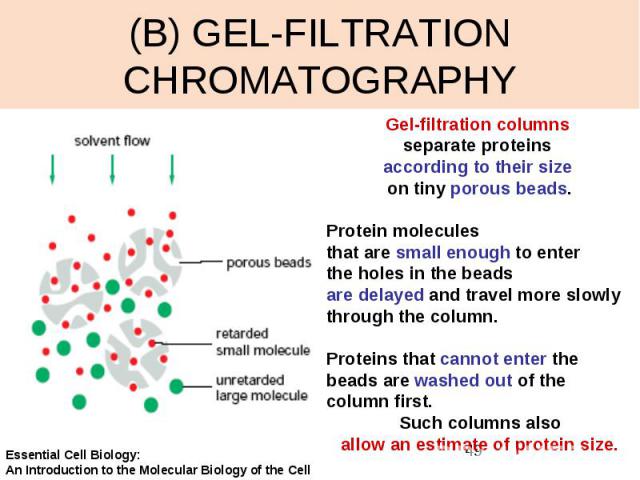
(B) GEL-FILTRATION CHROMATOGRAPHY
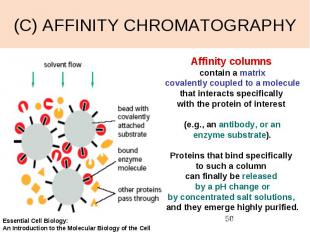
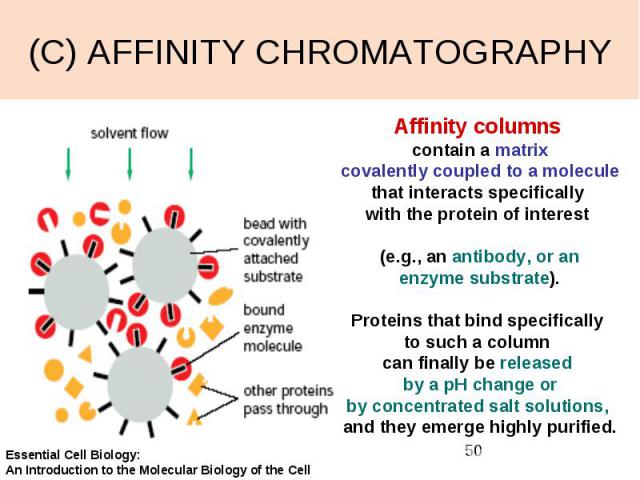
(C) AFFINITY CHROMATOGRAPHY

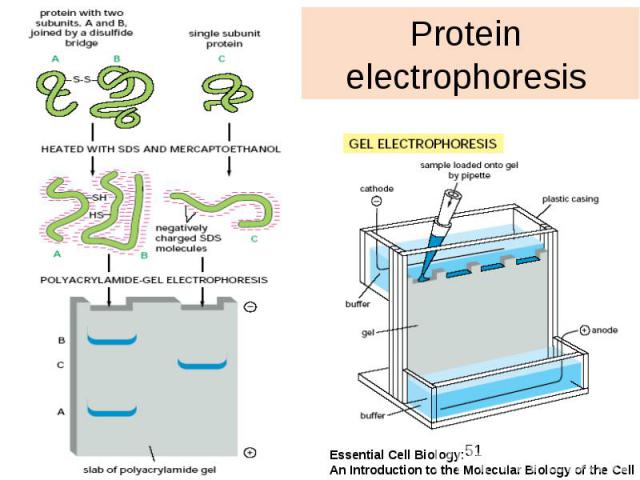
Protein electrophoresis
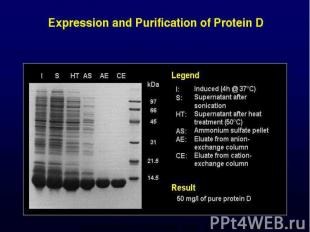
Fusion proteins increase production level facilitate purification (taq) detection of expression (GFP fusion) Redirection of proteins (secretion -> signal peptidases) Surface display (for screening of libraries) Tandem arrays (for small peptides, toxic proteins,..)
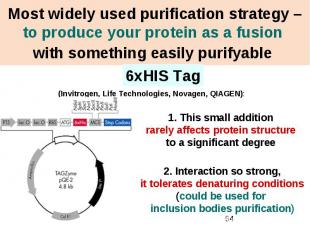
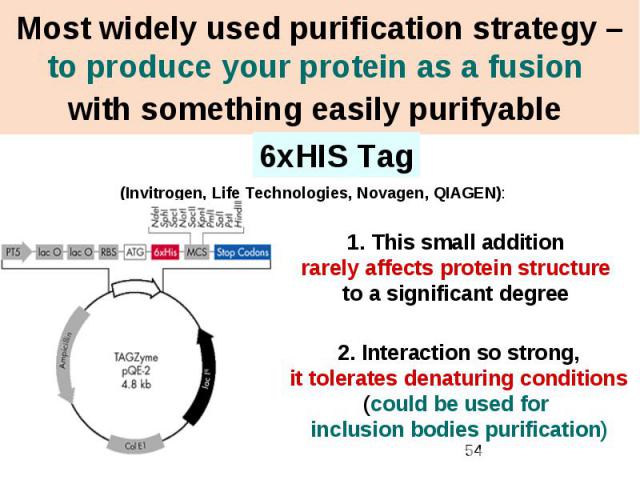
Most widely used purification strategy – to produce your protein as a fusion with something easily purifyable

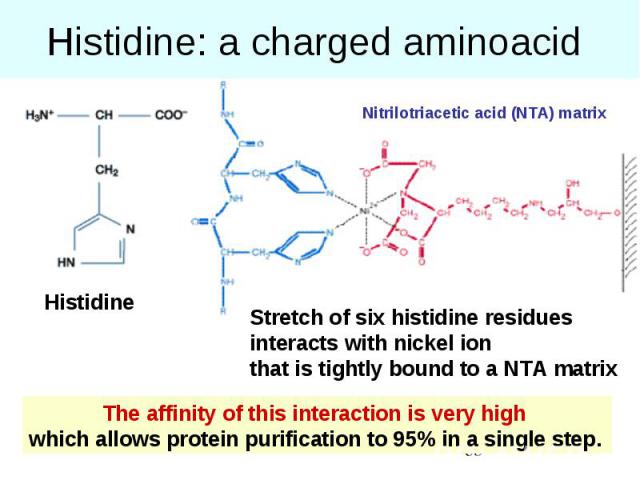
Histidine: a charged aminoacid
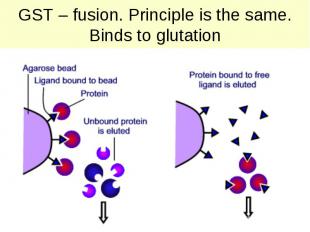
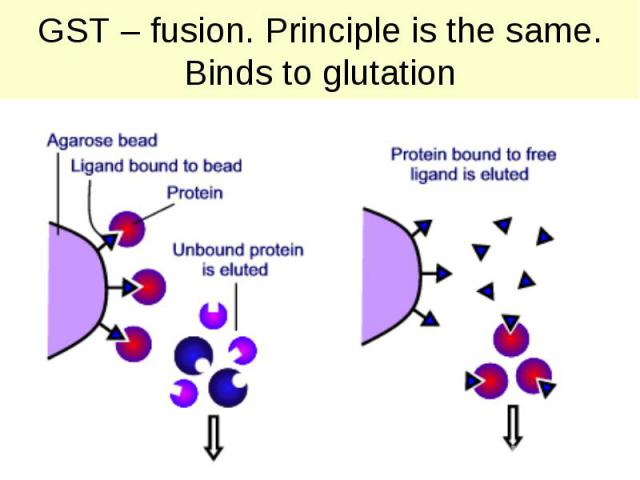
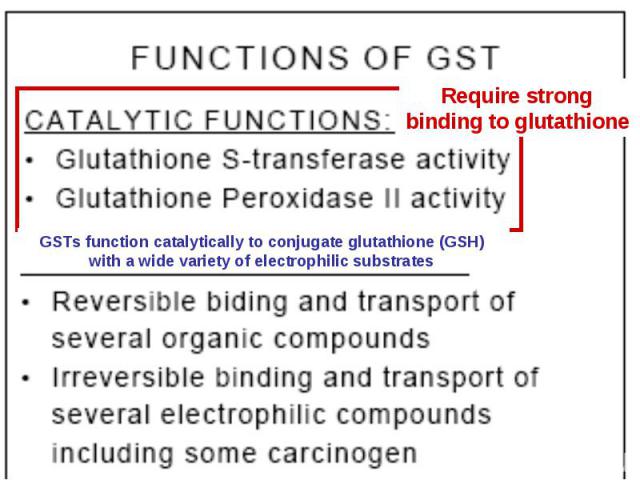
GST – fusion. Principle is the same. Binds to glutation

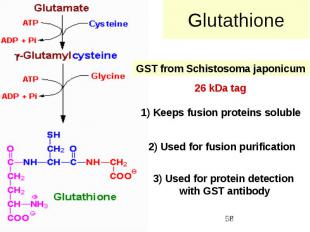
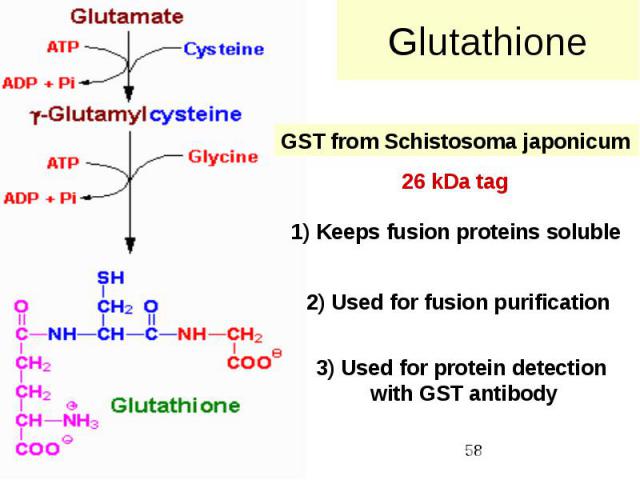
Glutathione
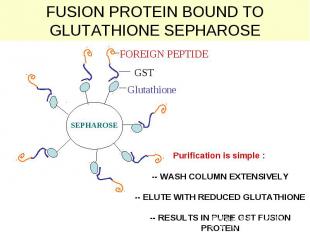
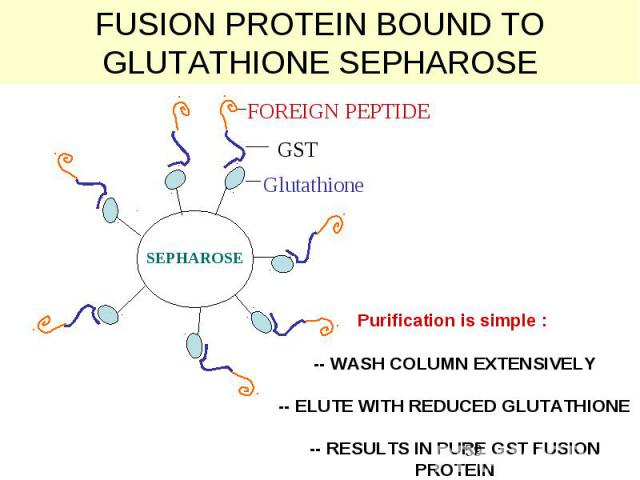
FUSION PROTEIN BOUND TO GLUTATHIONE SEPHAROSE
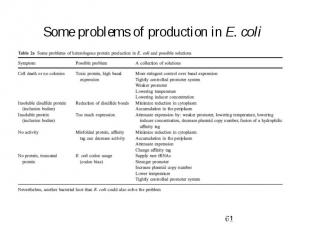
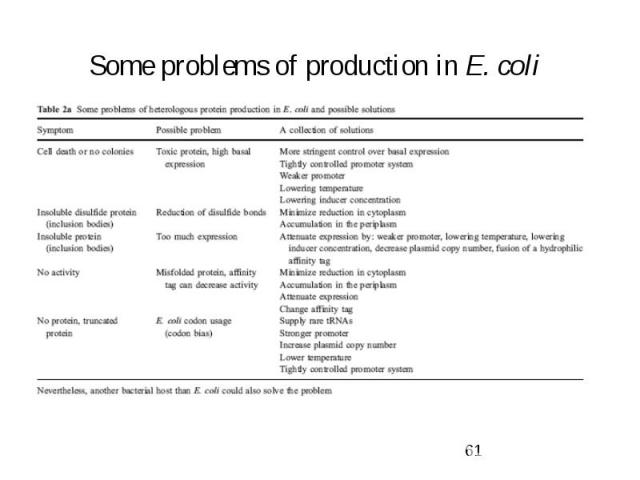
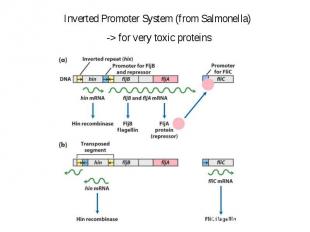
Some problems of production in E. coli
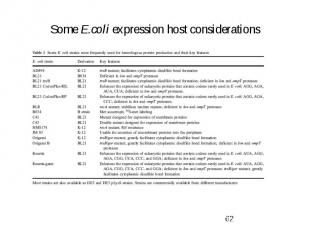
Some E.coli expression host considerations
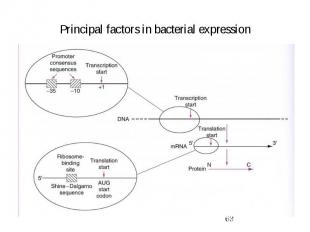
Principal factors in bacterial expression
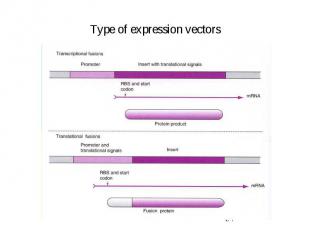
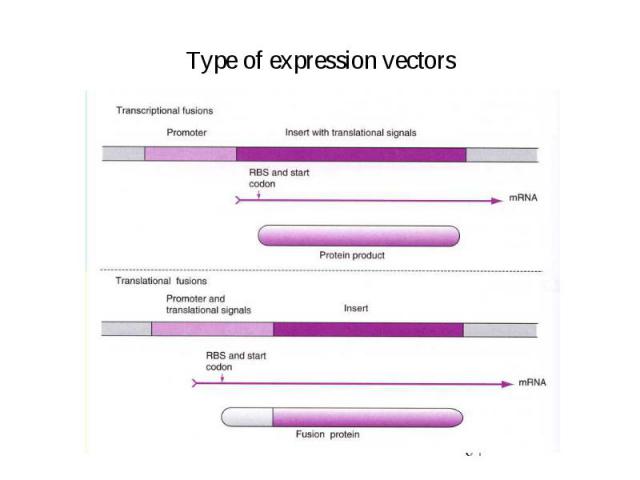
Type of expression vectors

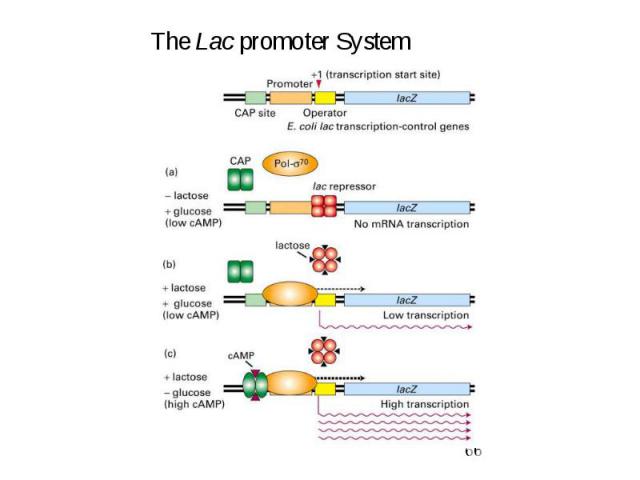
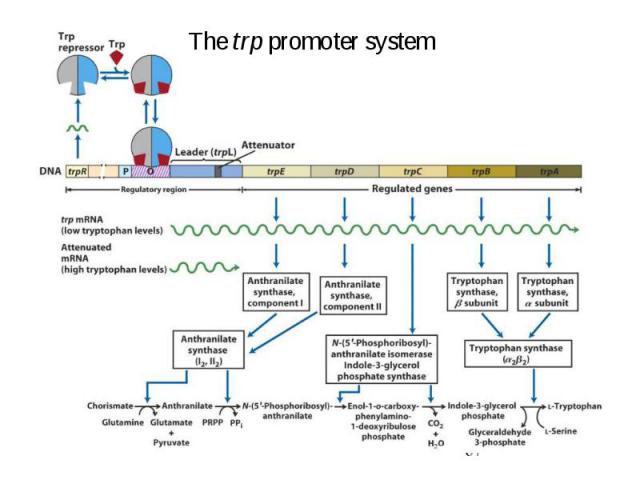
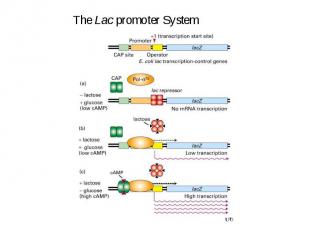
Initiation of Transcription Promoters for Expression in Prokaryotes In Escherichia coli - Lac system - plac - Trp system - synthetic systems – ptac, ptrc In Bacillus
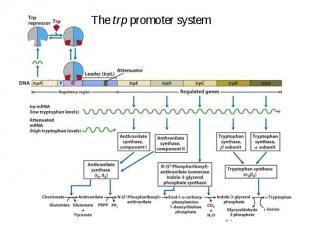
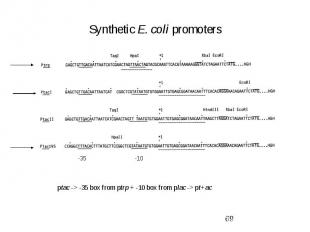
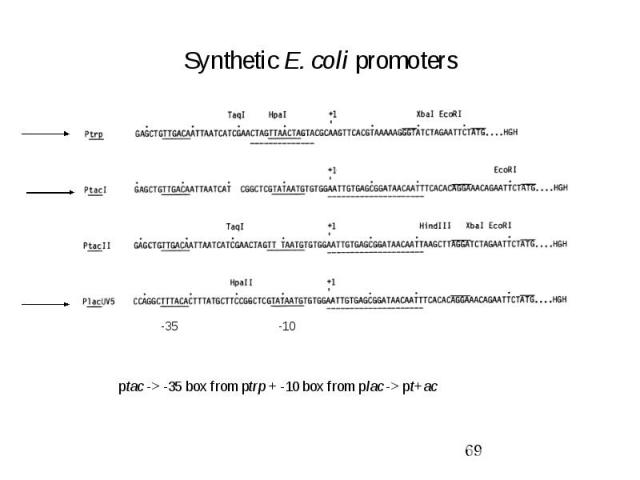
Synthetic E. coli promoters

Bacillus

Bacillus Antibiotic Producers: B. brevis (e.g. gramicidin, tyrothricin), B. cereus (e.g. cerexin, zwittermicin), B. circulans (e.g. circulin), B. laterosporus (e.g. laterosporin), B. licheniformis (e.g. bacitracin), B. polymyxa (e.g. polymyxin, colistin), B. pumilus (e.g. pumulin) B. subtilis (e.g. polymyxin, difficidin, subtilin, mycobacillin). Pathogens of Insects: B. larvae, B. lentimorbis, and B. popilliae are invasive pathogens. B. thuringiensis forms a parasporal crystal that is toxic to beetles. Pathogens of Animals: B. anthracis, and B. cereus. B. alvei, B. megaterium, B. coagulans, B. laterosporus, B. subtilis, B. sphaericus, B. circulans, B. brevis, B. licheniformis, B. macerans, B. pumilus, and B. thuringiensis have been isolated from human infections. The Genus Bacillus includes two bacteria of significant medical importance, B. anthracis, the causative agent of anthrax, and B. cereus, which causes food poisoning. Nonanthrax Bacillus species can also cause a wide variety of other infections, and they are being recognized with increasing frequency as pathogens in humans.

Bacillus Bacillus strains used as production organisms: - B. subtilis - B. brevis - B. licheniformis Transformation systems: - via competent cells (during transition from vegetative cells -> sporulation, cell can take up DNA (ss) when population reaches a metabolic state called competence) - protoplast - bacteriophage-mediated transduction Vectors: - replicating plasmids (pUB110, pE194, pC194, pHP13, shuttle vectors) -> replicating plasmids with temperature-sensitive origin of replication (replication stops above certain temp. -> pE194 stops above 45ºC) - integrative vectors (normally shuttle vectors) Promoters: - aprE promoter -> induction with onset of sporulation - amylase promoter -> growth-phase and nutrition regulated promoter (induction at end of exponential growth + repression by glucose) - sacB promoter (levansurase) -> not regulated - spac promoter -> hybrid promoter (subtilis phage + lac operator) -> induction with IPTG
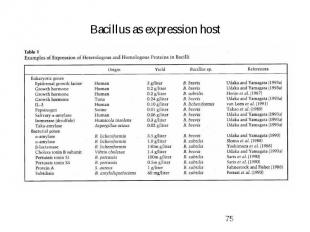
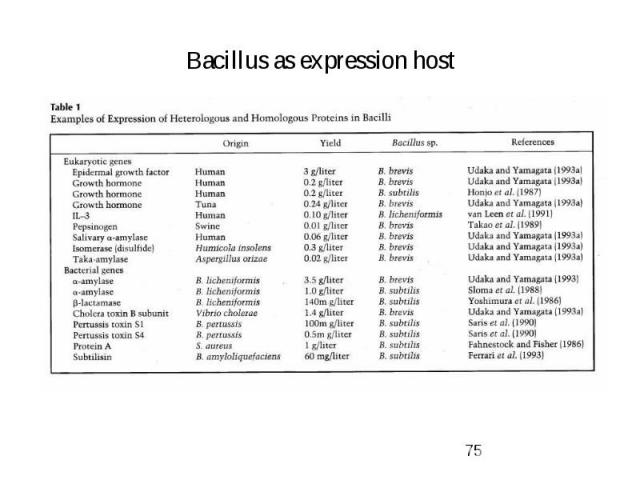
Bacillus as expression host
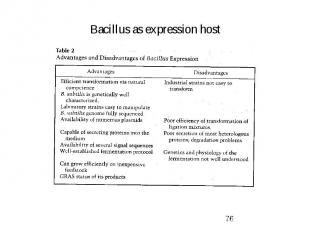
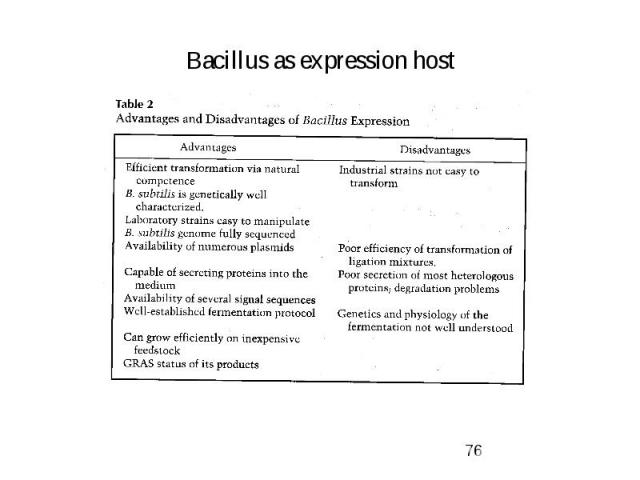
Bacillus as expression host

Products produced in Prokaryotic Systems Restriction Endonucleases -> produced in E. coli L- Ascorbic Acid (Vitamin C) -> recombinant Erwinia herbicola (gram-negative bacterium) Synthesis of Indigo (blue pigment -> dye cotton /jeans) -> produced in E. coli Amino Acids -> produced in Corynebacterium glutamicum (gram-positive bacterium) Lipases (laundry industry) -> from Pseudomonas alcaligenes produced in Pseudomonas alcaligenes Antibiotica (most of them from Streptomyces, other gram-positive bacteria, fungi) -> produced in recombinant Streptomyces and fungi (Penicillium) Biopolymers (PHB -> biodegradable plastics) -> produced in E. coli (stabilized with parB)

Expression in Eukaryotic Systems Yeast - Saccharomyces cerevisiae (baker’s yeast) - Pichia pastoris Insect Cells – Baculovirus Mammalian Cells
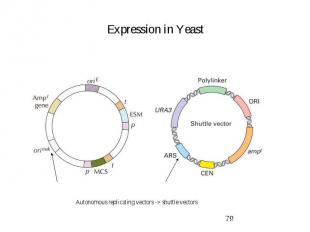
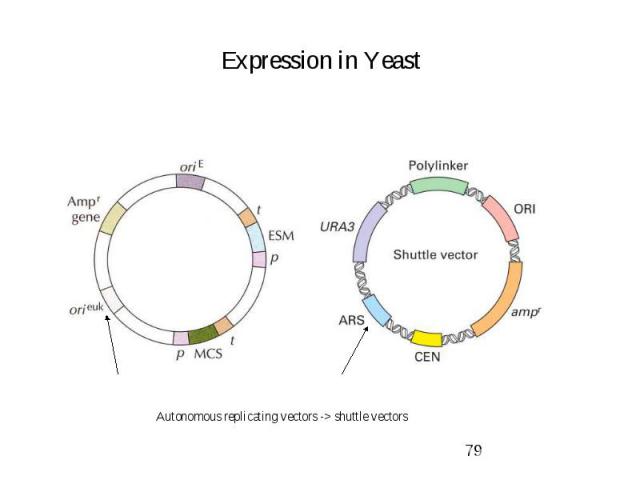
Expression in Yeast
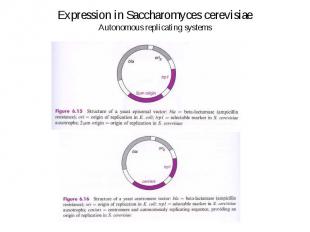
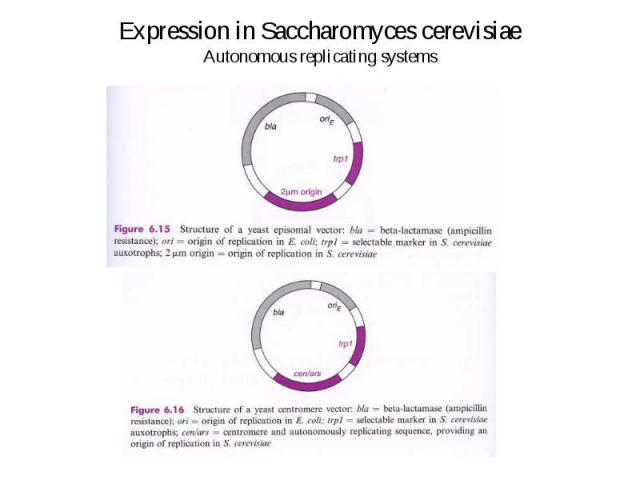
Expression in Saccharomyces cerevisiae Autonomous replicating systems
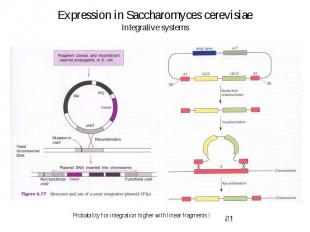
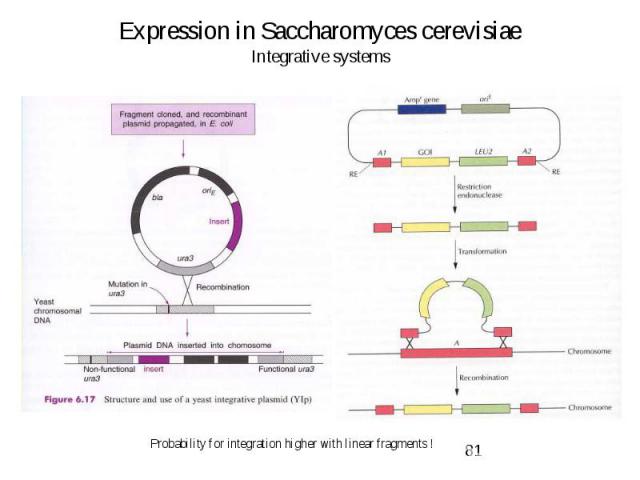
Expression in Saccharomyces cerevisiae Integrative systems
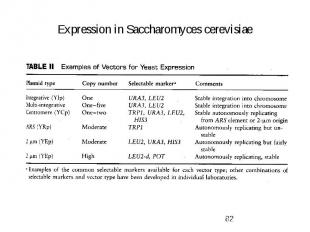
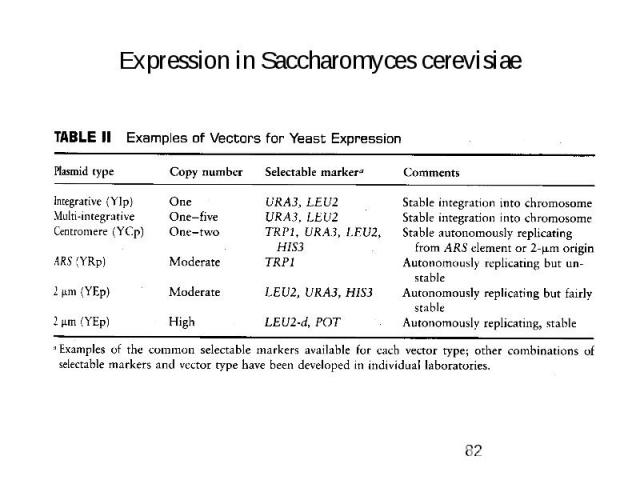
Expression in Saccharomyces cerevisiae

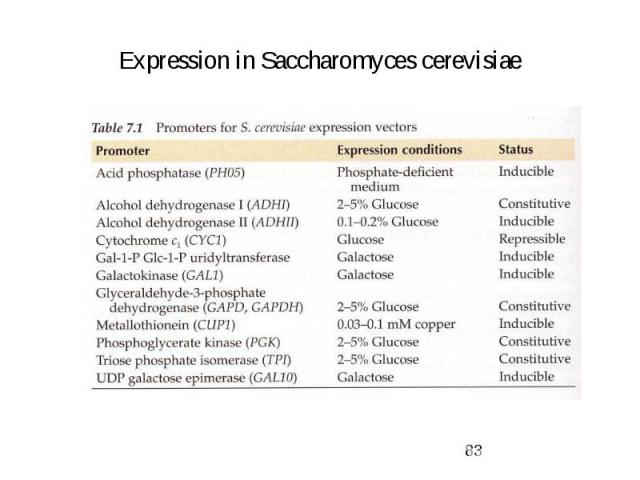
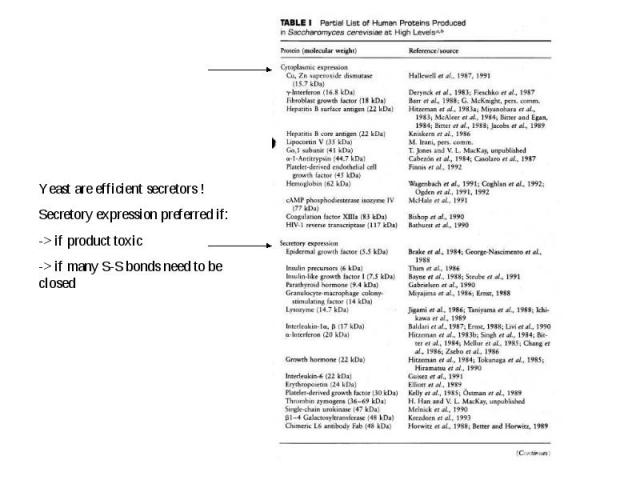
Expression in Saccharomyces cerevisiae

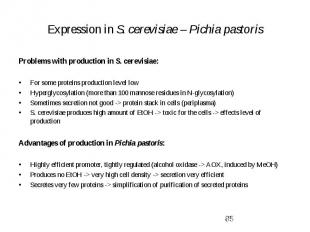
Expression in S. cerevisiae – Pichia pastoris Problems with production in S. cerevisiae: For some proteins production level low Hyperglycosylation (more than 100 mannose residues in N-glycosylation) Sometimes secretion not good -> protein stack in cells (periplasma) S. cerevisiae produces high amount of EtOH -> toxic for the cells -> effects level of production Advantages of production in Pichia pastoris: Highly efficient promoter, tightly regulated (alcohol oxidase -> AOX, induced by MeOH) Produces no EtOH -> very high cell density -> secretion very efficient Secretes very few proteins -> simplification of purification of secreted proteins
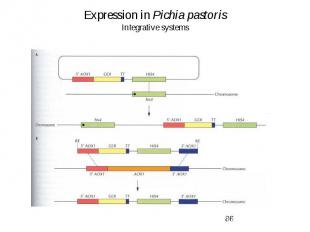
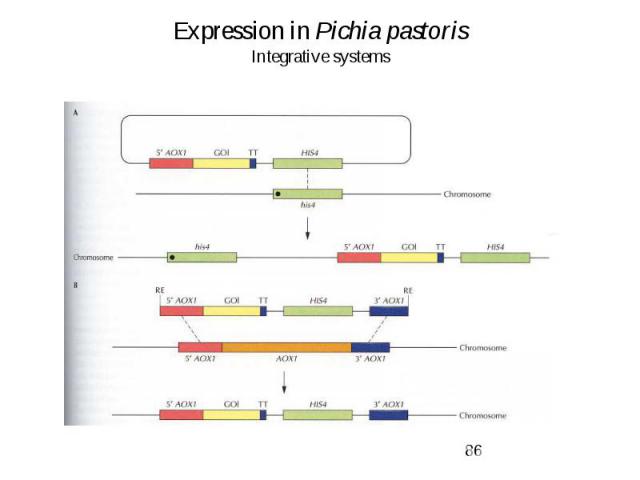
Expression in Pichia pastoris Integrative systems
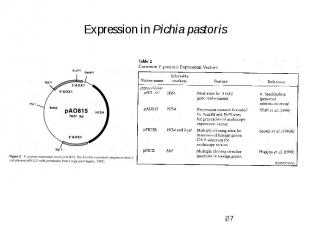
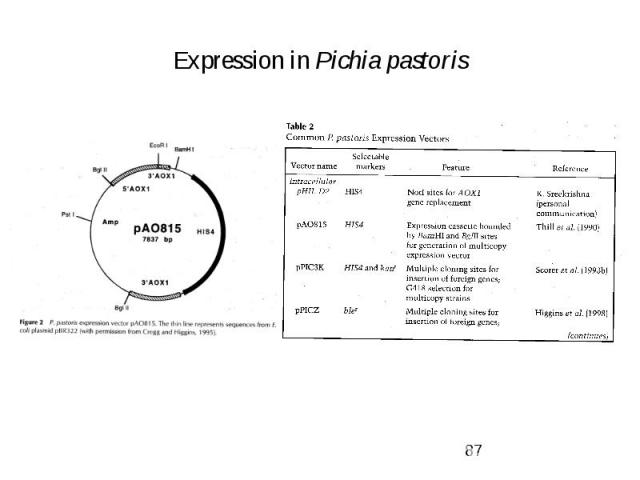
Expression in Pichia pastoris

Expression in Pichia pastoris
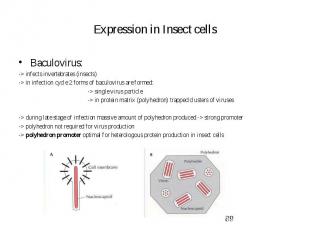
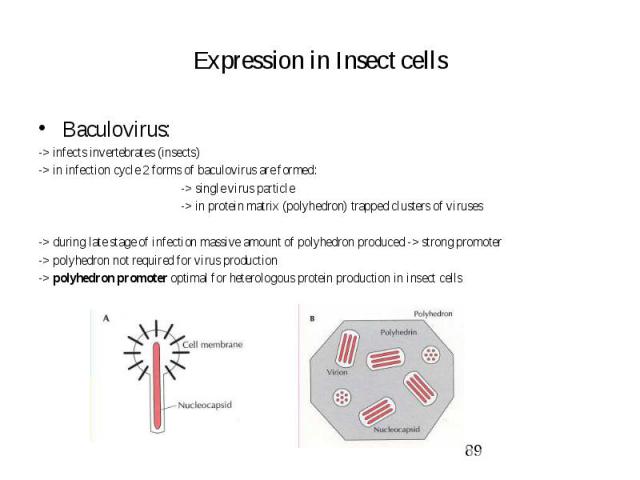
Expression in Insect cells Baculovirus: -> infects invertebrates (insects) -> in infection cycle 2 forms of baculovirus are formed: -> single virus particle -> in protein matrix (polyhedron) trapped clusters of viruses -> during late stage of infection massive amount of polyhedron produced -> strong promoter -> polyhedron not required for virus production -> polyhedron promoter optimal for heterologous protein production in insect cells
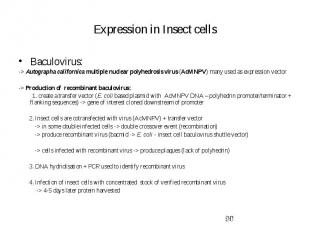
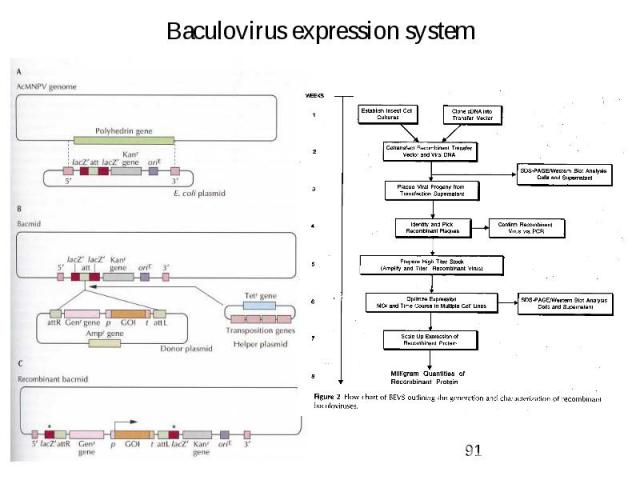
Expression in Insect cells Baculovirus: -> Autographa californica multiple nuclear polyhedrosis virus (AcMNPV) many used as expression vector -> Production of recombinant baculovirus: 1. create a transfer vector (E. coli based plasmid with AcMNPV DNA – polyhedrin promoter/terminator + flanking sequences) -> gene of interest cloned downstream of promoter 2. Insect cells are cotransfected with virus (AcMNPV) + transfer vector -> in some double infected cells -> double crossover event (recombination) -> produce recombinant virus (bacmid -> E. coli - insect cell baculovirus shuttle vector) -> cells infected with recombinant virus -> produce plaques (lack of polyhedrin) 3. DNA hydridisation + PCR used to identify recombinant virus 4. Infection of insect cells with concentrated stock of verified recombinant virus -> 4-5 days later protein harvested
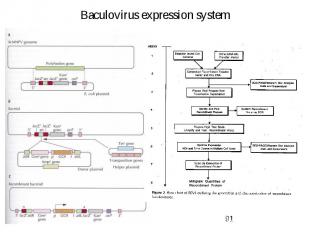
Baculovirus expression system

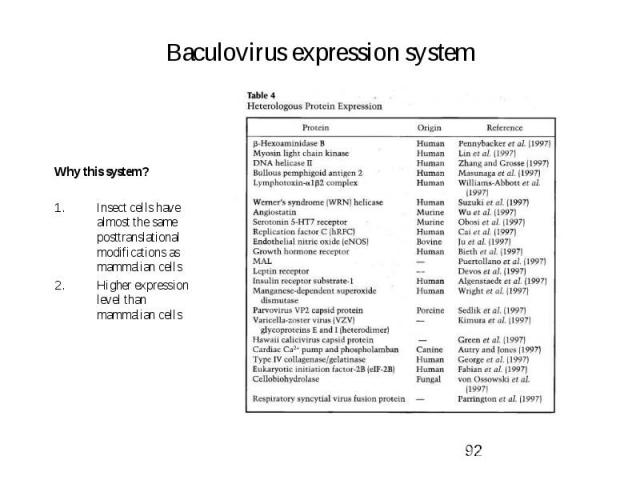
Baculovirus expression system Why this system? Insect cells have almost the same posttranslational modifications as mammalian cells Higher expression level than mammalian cells

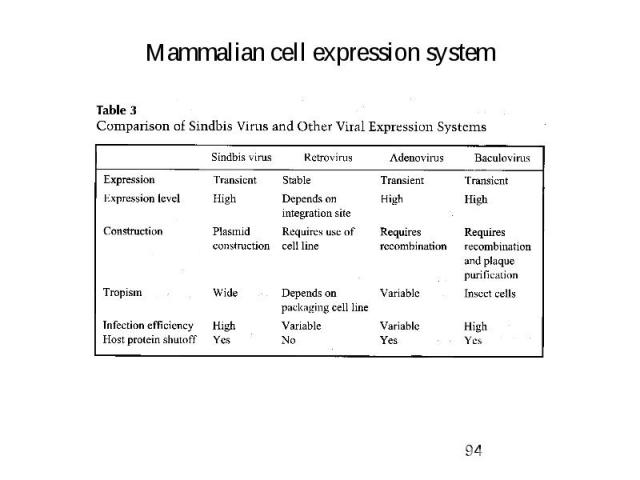
Mammalian cell expression system 1. Why do we use that system? -> to get full complement of posttranslational modifications on proteins 2. Developed cell lines: -> short term (transient) expression -> autonomous replicating systems -> viral origins (SV40) - African green monkey kidney (COS) - baby hamster kidney (BHK) - human embryonic kidney (HEK-239) -> long term (stable) expression -> integration into chromosome -> viral origins - chinese hamster ovary (CHO)
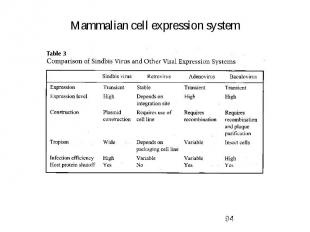
Mammalian cell expression system

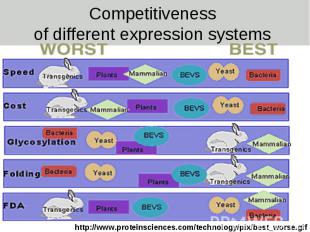
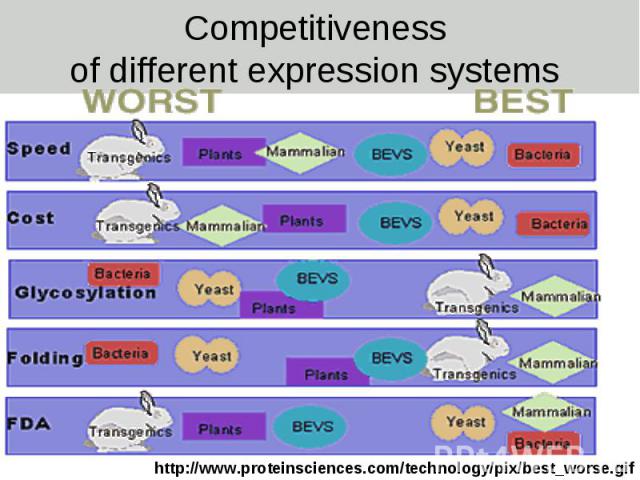
Competitiveness of different expression systems